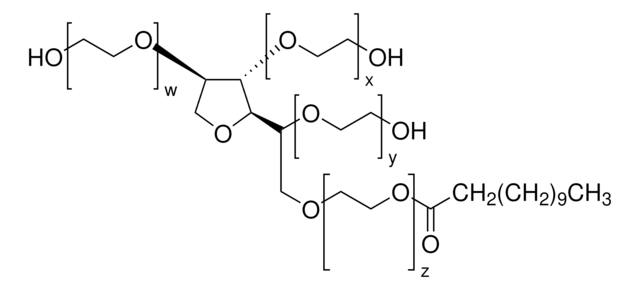
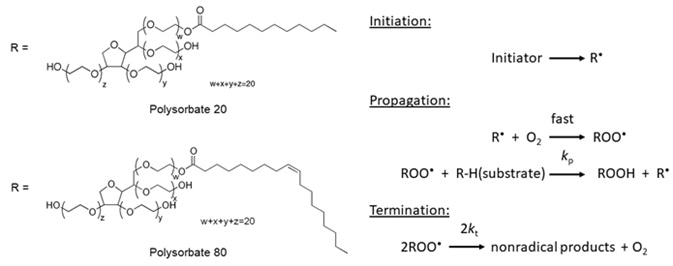
Polysorbate 20 Degradation in Biopharmaceutical Formulations

Pharmaceutics, Free Full-Text

Polysorbate 20 Degradation in Biopharmaceutical Formulations: Quantification of Free Fatty Acids, Characterization of Particulates, and Insights into the Degradation Mechanism
View of ANALYSIS OF POLYSORBATE 80 SOLUTION STABILITY UNDER STRESS CONDITIONS TO ENSURE ITS QUALITY AS A BIOPHARMACEUTICAL EXCIPIENT

Formulation mitigations for particle formation induced by enzymatic hydrolysis of polysorbate 20 in protein-based drug products: insights from a full-factorial longitudinal study, AAPS Open

Full article: Prediction of long-term polysorbate degradation according to short-term degradation kinetics
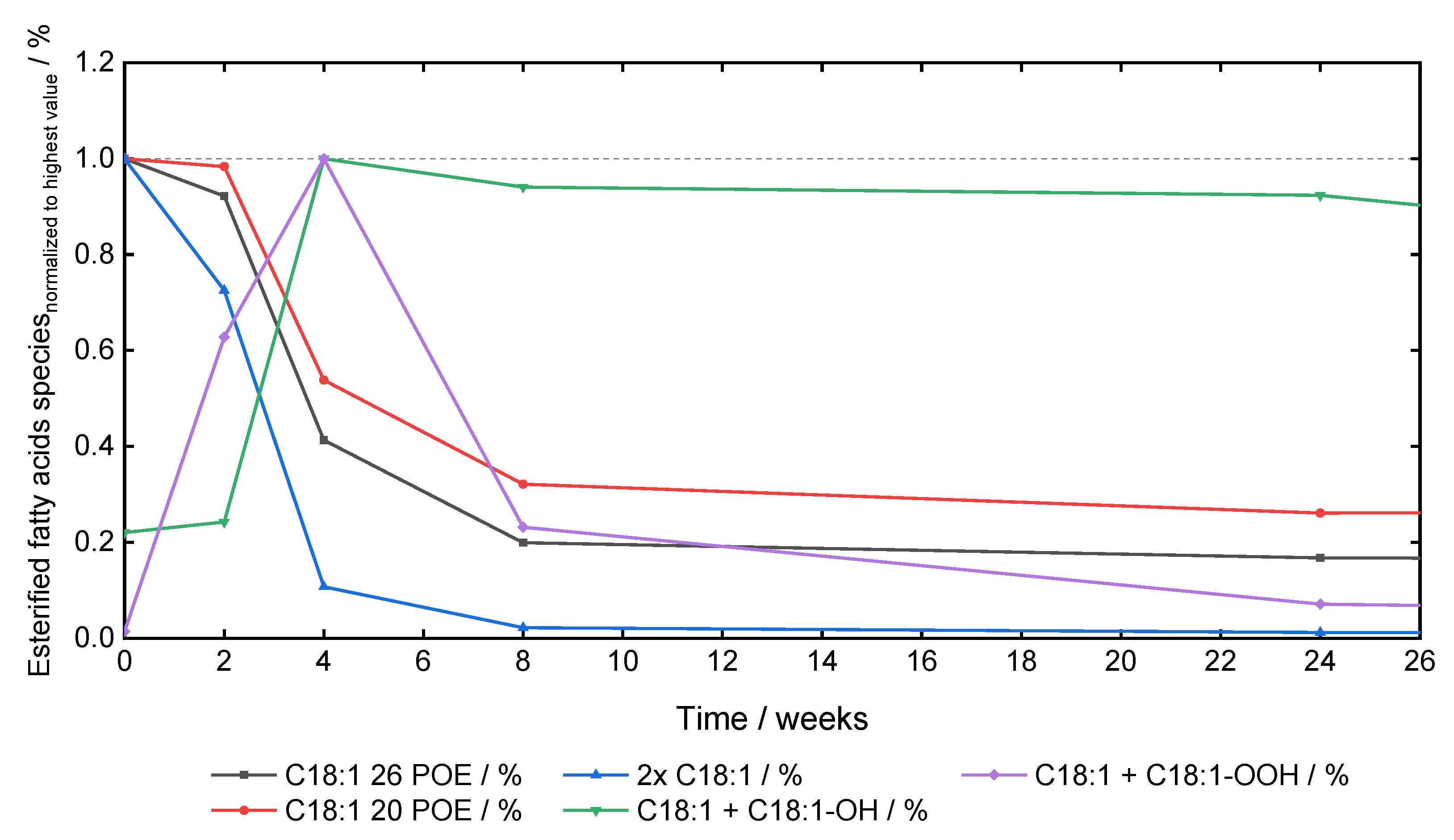
Pharmaceutics, Free Full-Text

Profiling Active Enzymes for Polysorbate Degradation in Biotherapeutics by Activity-Based Protein Profiling

Molecules, Free Full-Text

Understanding Particle Formation: Solubility of Free Fatty Acids as Polysorbate 20 Degradation Byproducts in Therapeutic Monoclonal Antibody Formulations.

Molecules, Free Full-Text
View of ANALYSIS OF POLYSORBATE 80 SOLUTION STABILITY UNDER STRESS CONDITIONS TO ENSURE ITS QUALITY AS A BIOPHARMACEUTICAL EXCIPIENT
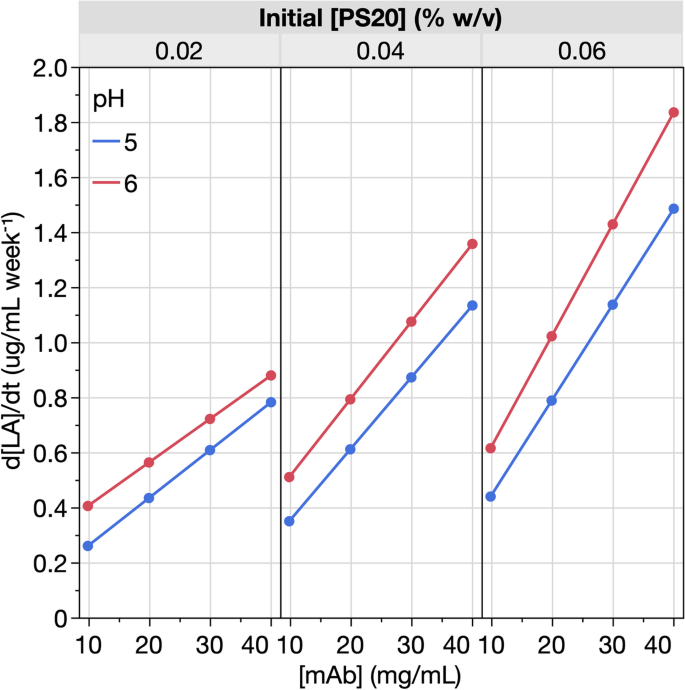
Formulation mitigations for particle formation induced by enzymatic hydrolysis of polysorbate 20 in protein-based drug products: insights from a full-factorial longitudinal study, AAPS Open

Identification of the specific causes of polysorbate 20 degradation in monoclonal antibody formulations containing multiple lipases

Identification of Subvisible Particles in Biopharmaceutical Formulations Using Raman Spectroscopy Provides Insight into Polysorbate 20 Degradation Pathway. - Abstract - Europe PMC