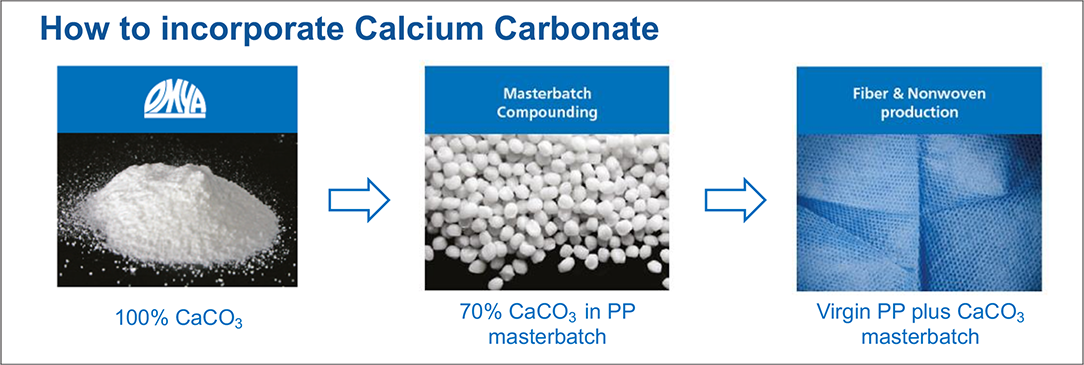
Magnesium Impurities Decide the Structure of Calcium Carbonate Hemihydrate
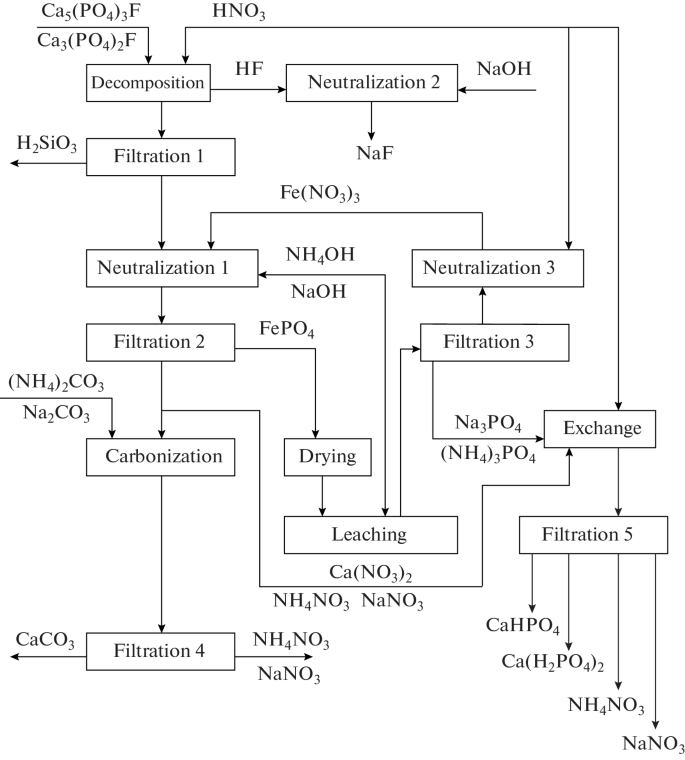
Theoretical and Practical Foundations of the Selective Separation of Phosphate Ions from Phosphate Ores with a High Content of Iron Impurities with the Recirculation Method

Arrhenius plot for the nucleation and crystal growth of aragonite

Insect frass as a substrate to stimulate native ureolytic bacteria for microbial-induced carbonate precipitation in soil biocementation

Julie AUFORT, Researcher, PhD, French National Centre for Scientific Research, Paris, CNRS, Institut de Minéralogie et de Physique des Milieux Condensés (IMPMC)
Crystals, Free Full-Text

PDF] Proto-calcite and proto-vaterite in amorphous calcium carbonates.

Pressure-driven fusion of amorphous particles into integrated monoliths

Julie AUFORT, Researcher, PhD, French National Centre for Scientific Research, Paris, CNRS, Institut de Minéralogie et de Physique des Milieux Condensés (IMPMC)

PDF) Magnesium Impurities Decide the Structure of Calcium Carbonate Hemihydrate

Conceptual flowsheet making use of magnesium sulphate crystallization

Rationally designed calcium carbonate multifunctional trap for contaminants adsorption - ScienceDirect

Raffaella DEMICHELIS, Senior Lecturer & ARC Future Fellow

Synthetic amorphous calcium phosphates (ACPs): preparation, structure, properties, and biomedical applications - Biomaterials Science (RSC Publishing) DOI:10.1039/D1BM01239H

Raffaella DEMICHELIS, Senior Lecturer & ARC Future Fellow

Hydration reactivity difference between dicalcium silicate and tricalcium silicate revealed from structural and Bader charge analysis