Exploring the Fascinating World of Prussian Blue
Discover the artistic applications of Prussian Blue, a pigment with a rich heritage and enduring appeal. Explore its deep blue hues and the science behind its creation
Arte Fo is a leading company that sell High-Quality selection of Ceramic Pottery glaze Shop. Variety of Pottery Paint, Underglazes colors, Pottery Glazes, Mix Glaze Recipes, Art Supplies & Materials Shop
Fe4[Fe(CN)6]3 Prussian Blue is a deep blue pigment that has been used in various applications, including art, chemistry, and medicine. Here's a brief overview of what Prussian Blue is and its common uses:Chemical Composition: Prussian Blue is a synthetic pigment with the chemical formula Fe7(CN)18. It is known by several other names, including Berlin Blue and, historically, Parisian Blue.Color and Appearance: Prussian Blue is characterized by its deep, dark blue color, often with a slightly greenish or purple tint. It is a dense and fine-grained powder that can be mixed with binders to create paints and dyes.Historical Significance: Prussian Blue was discovered in the early 18th century by a Berlin-based paintmaker named Diesbach. Its creation marked a significant breakthrough in the development of synthetic pigments and had a profound impact on art and other fields.Artistic Use: Prussian Blue has been widely used in the world of art as a pigment for oil and watercolor paints. It is known for its vibrant, long-lasting color and has been used by many famous artists throughout history.Chemical Reactions: Prussian Blue has been used in various chemical reactions, particularly in analytical chemistry. It is known for its ability to form complex compounds with certain metal ions, making it useful for detecting and quantifying these ions in analytical procedures.Medicine: Prussian Blue has been employed in medicine as a treatment for heavy metal poisoning, specifically for thallium, cesium, and radioactive isotopes of cesium and strontium. It works by binding to these substances, allowing them to be excreted from the body.Prussian blue's ability to incorporate monovalent metallic cations (Me+) makes it useful as a sequestering agent for certain toxic heavy metals. Pharmaceutical-grade Prussian blue, in particular, is used for people who have ingested thallium (Tl+) or radioactive caesium (134Cs+, 137Cs+) . According to the International Atomic Energy Agency (IAEA), an adult male can eat at least 10 g of Prussian blue per day without serious harm. The U.S. Food and Drug Administration (FDA) has determined the "500-mg Prussian blue capsules, when manufactured under the conditions of an approved New Drug Application, can be found safe and effective therapy" in certain poisoning cases. Radiogardase (Prussian blue insoluble capsules) is a commercial product for the removal of caesium-137 from the intestine, so indirectly from the bloodstream by intervening in the enterohepatic circulation of caesium-137, reducing the internal residency time (and exposure) by about two-thirds. In particular, it was used to adsorb and to remove 137Cs+ from the organism of those poisoned in the Goiânia accident in Brazil.Photography: In historical photography processes, Prussian Blue was used as a photosensitive material.Dyeing: Prussian Blue has been used in the textile industry as a dye for fabrics.Household use: Prussian blue is present in some preparations of laundry bluing, such as Mrs. Stewart's Bluing.Battery materials: Prussian blue (PB) has been studied for its applications in electrochemical energy storage and conversion since 1978. It is possible to replace the Fe metal centers in PB with different metal ions such as Mn, Co, Ni, Zn, etc. to form electrochemically active Prussian blue analogues (PBAs). PB/PBAs and their derivatives can be used as electrode materials for reversible alkali-ion insertion and extraction in lithium-ion battery, sodium-ion battery, and potassium-ion battery.It's important to note that Prussian Blue has been replaced by alternative pigments in some applications due to its potential toxicity and lightfastness issues. Additionally, some people may be sensitive or allergic to it. While Prussian Blue has a rich history and continues to be used in various niche applications, the use of other blue pigments, such as synthetic ultramarine and phthalo blue, has become more common in contemporary art and industry.Perhaps the most striking example of van Gogh's use of Prussian blue is Starry Night (1889)Formula: Fe4[Fe(CN)6]3Molar Mass: 859.23 g/molForm: Blue opaque crystalsCAS Number: 14038-43-8EC Number: 237-875-5Density: 1.8 g/cm³Synonyms: Prussian blue, Pigment Blue 27, Tiemans Soluble Blue, FERRIC FERROCYANIDE, Iron Blue, iron(III) hexacyanoferrate(II), Ferrocin, Parisian blue, Berlin Blue, Ferric hexacyanoferrate, Preussischblau, Turnbulls Blau, Berliner Blau, iron(2+);iron(3+);octadecacyanide, Milori blue, Midnight blue, Brandenburg blue, Sarum blue, iron (III) ferrocyanide

Multifunctional Prussian blue analogue magnets: Emerging opportunities - ScienceDirect

Barn Cottage Records
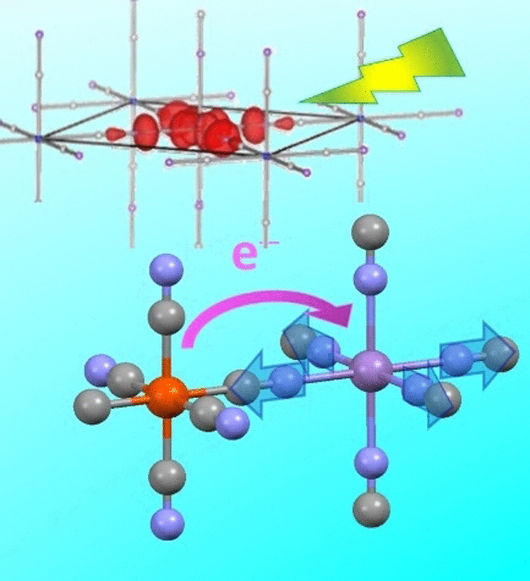
Electrons Passed Around - Ultrafast charge transfer in Prussian blue analogues
Prussian blue - Wikipedia

Prussian Blue (1947) by Anne Hocking – Dead Yesterday

Simple Galaxy Painting with 3D Mountain Crafting, Umashree Taparia

Prussian Blue: Artist Explores the Color of the Holocaust - The Media Line
The twelfth book in the Sunday Times and New York Times bestselling series, perfect for fans of John le Carre and Robert Harris. 'One of the greatest

Prussian Blue

The History of Prussian Blue - Jackson's Art Blog

Making of Prussian Blue: Meditations on Colour

Prussian Blue: Chemistry, Commerce, and Colour in Eighteenth‐Century Paris - Guichard - 2023 - Art History - Wiley Online Library